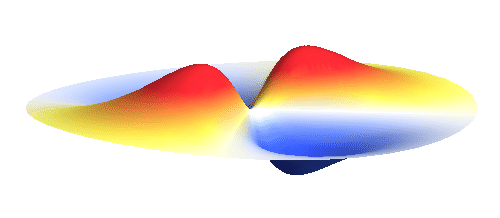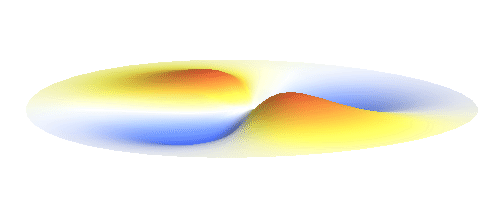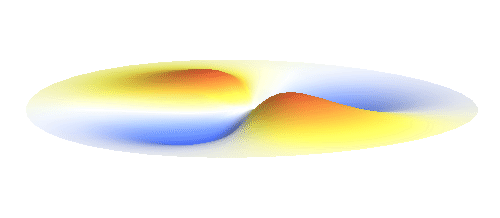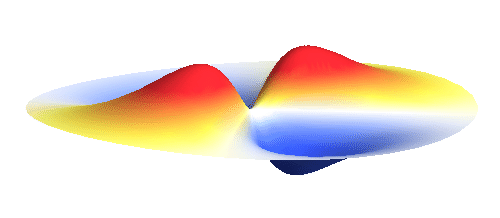Consider the subtleness of the sea; how its most dreaded creatures glide under water, unapparent for the most part, and treacherously hidden beneath the loveliest tints of azure.
— Herman Melville, Moby-Dick; or, The Whale
Erwin with his psi can do
Calculations quite a few.
But one thing has not been seen:
Just what does psi really mean?— Erich Hückel’s unpublished poem
You may find the mathematical formulation of mechanics abstruse, but all of us intuitively understand how it works. You know that you have to run fast for bus if you leave home too late, you understand why objects cast longer shadows at sunset than they do at noon, and you commonly use a lever to lift objects that are otherwise to heavy to be moved. The evolution has shaped us to understand velocities, angles and forces. We are all physicists at heart. But we are classical physicists and our intuition fails us when we encounter the world of the small.
Quantum mechanics is based on a handful of postulates. They are strange, but they are rigorously formulated and they passed all experimental tests ever devised so they have to be accepted as such. In the following text we will discuss some of them. To avoid ambiguity I will discuss an electron, but you should keep in mind that these laws apply to all the particles down there.
You have certainly heard about at least some of the strange conclusions of quantum mechanics, like “an electron can be at several places at once” or that “we cannot measure the position and velocity of an electron at the same time“. These statements are true and indeed odd. But they are much less perplexing if we accept one experimental fact: Electrons are not really point-like particles. They are better to be thought of as waves. These waves occupy some finite region of space as a cloud does, and as well as a cloud these waves do not have sharp boundaries. A typical extent of these waves is usually microscopic. In fact, it is the extent of these electron waves that defines the size of an atom.
To appreciate the idea of an electron wave, we have to address two questions. First, what is the evidence that they really are waves? And second, how does this waviness mitigate those baffling claims mentioned above? Let us start with the experimental question. Though the concept of an electron wave had been around since de Broglie proposed it in 1924 to explain properties of emission spectra of hydrogen, it became much more tangible and inescapable after an experiment performed by Claus Jönsson in 1961. His experiment was voted “the most beautiful experiment” by the readers of Physics World in 2002. He brought the world of the small much closer to us. But to explain its consequences we have to return to the beginning of 19th century when a far-reaching discovery happened in another field of physics — the study of light.
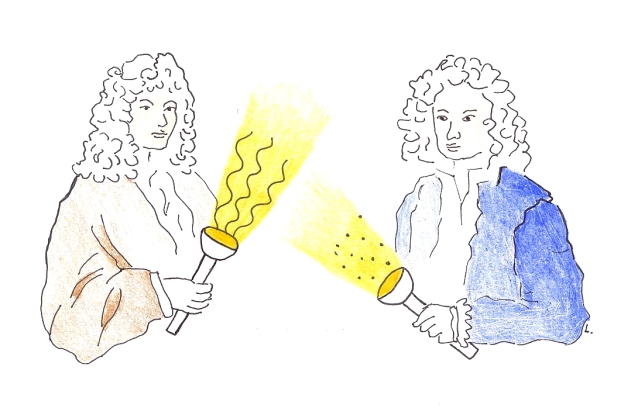
Since the time of Isaac Newton and Christiaan Huygens there had been a discord in the physics community about the character of light: Is it made of particles or waves? After more than a century, Thomas Young resolved this dispute by performing an experiment presently known as “the double-slit experiment”. The setting was easy. Consider the propagation of light from a light bulb to a wall and also some objects standing in the way. That’s all Young had to study to reach his conclusion. He just used a very special object as an obstacle — a dark foil with two parallel narrow openings. The “double-slit”.
You probably visualize light moving in straight lines (the light rays) so you understand why objects standing in the way cast shadows on the wall. If the light bulb is very tiny, all rays leave the bulb at more-or-less one spot and you expect the shadows to be sharp. This is what the Newton’s particle theory predicts: The particles of light move in straight lines unless an object stands in the way. On the other hand, the prediction of Huygens’ wave assumption is different. Waves can change direction of motion when they pass by a corner. For example, when you are playing outside, you can hear people chattering and a car engine running on the other side of a building in spite of the fact that there is no straight line connecting those events to your ear. It works because sound is a wave and it does not propagate in straight “rays”.
So Thomas Young put a double-slit in front of the bulb and looked at the shadow. And, lo and behold, he didn’t see two bright strips. Instead he observed a multitude of bright and dark strips! This contradicts the particle theory, while the wave theory has no troubles to explain it. The phenomenon is called wave interference. It is a bit awkward to be described in words so I urge you: Please watch this neat video where wave interference is demonstrated with both light and water waves. (An important remark: The reason that we do not perceive the wave character of light in our everyday experience is its minute wavelength — about one hundredth of the thickness of your hair. In Young’s experiment the two slits have to be very close to each other.)
Now let’s move back to 1961. Claus Jönsson did the same experiment with a beam of electrons. He looked at the wall and, voilà, found an interference pattern too! Even more fascinating is that this experiment can be carried out with such weak electron beam that there is at each moment just one electron between the source and the wall. Even then one would still find the interference pattern. This means that every electron is a wave by itself, this wave goes through both of the slits and then interferes with itself. There is really no contradiction. In his celebrated physics lectures Feynman concludes that “the paradox is only a conflict between reality and your feeling of what reality ought to be.” Meanwhile, analogous experiments have been performed with other particles as well, the largest up-to-date being fullerene (1 300 000 times heavier than electron) and a derivative of phthalocyanine (I don’t know what that is either, but it is 2 400 000 times heavier than electron).
Erwin Schrödinger, the guy mentioned in the Hückel’s poem, devised an equation that describes propagation of these waves. From mathematical point of view it is not precisely a wave equation, but it is harmless for us now to regard it as such. So, for example, if you put an electron next to a proton, it can get trapped due to the attractive electrostatic interaction. This has to be viewed as a wave localized around the proton, not leaking to the outer space, and somehow oscillating in time.
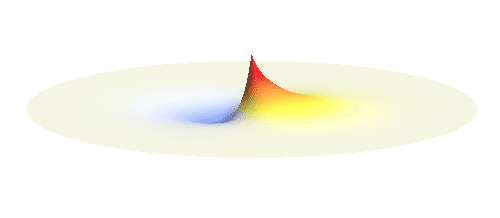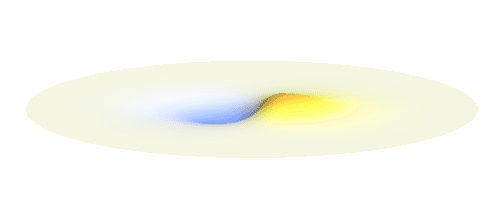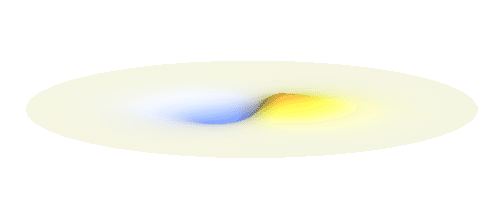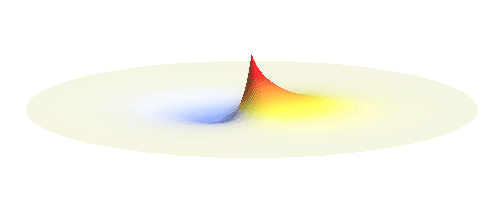
An important concept is that of a standing wave which is often explained using an elastic string. In similar way the electron waves can also be “standing”. Do you remember the chemistry class where we denoted various electron states (usually called orbitals) by strange combinations of symbols like 1s, 2s, 2p_x or 3d_xy? They are just labels for all possible standing waves of an electron in a hydrogen atom. The following four animations display the four aforementioned waves in the xy-plane. (Keep in mind that these waves actually oscillate in three dimensions but that is too difficult to fit into your two-dimensional computer screen. A remark for experts: Of course I only plot the real component of the wave.)
Notice also that the frequency of these standing waves is different. The first picture has the longest period, i.e. it oscillates very lazily, while the last one is very dynamic. It turns out that the rate of these oscillations is proportional to the electron energy. Since there is just a discrete set of various standing waves, the electron in this system can possess only certain discretized values of energy. That is a well-known experimental observation that de Broglie originally intended to explain.
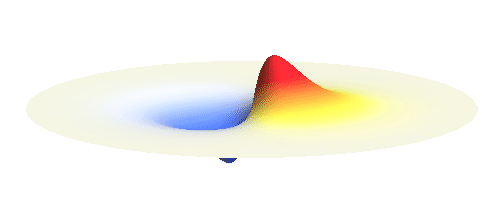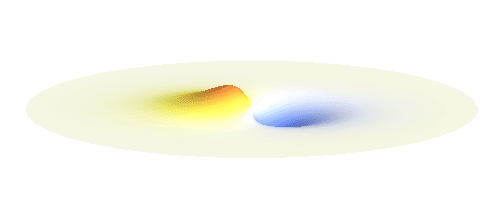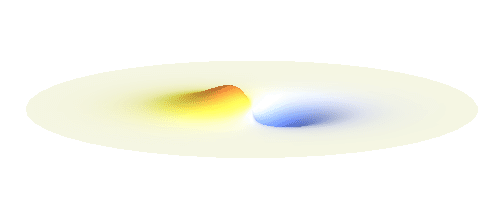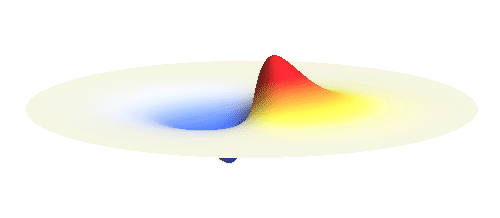
Now we must inevitably arrive at two of the postulates of quantum mechanics. First, an actual electron wave can be any combination of those standing waves mentioned above. This is not very surprising since all waves can combine in a similar way. If you listen to an orchestra, all sound waves coming from all the instruments come to your ear combined together and you can perceive all the beauty at once. Waves on a water pond can add together in the same way. This is just what waves do and the electron wave is no exception. A particular combination of 1s and 2p_x orbitals might look like the following animation.
The second postulate is about measurement. This is certainly the most troubling of the postulates. Since electrons are so small, it is not easy to devise an experiment that probes its properties. For simplicity, think of a measurement of energy as asking the electron “Hey, buddy, what is your energy?” upon which it willingly gives some number. When the electron wave is one of the standing ones mentioned above, there is no complication: The electron gives you a number and continues its existence without bothering about you.
However, a complication occurs when the electron wave happens to be a combination of several of the standing waves. A sober person would expect that the electron would tell you some average of the energies of the corresponding waves. But this is not what happens. The electron randomly chooses energy of one of those waves and after the measurement it instantaneously “collapses” into that particular wave. The measurement changes the state of the electron and there is no way to circumvent this problem. It is as if you tried to localize a car by setting a lot of mines on the road, instead of innocently looking at it from a distance. In quantum mechanics every measurement “undermines” the electron leading to a change of its state. This strange characteristic of measurement troubles physicists until today, see e.g. here and here.
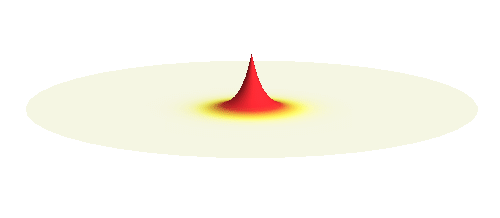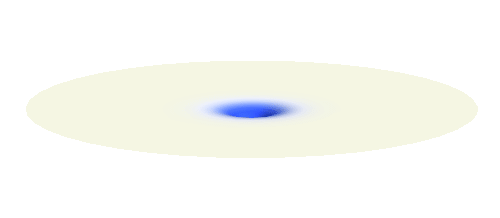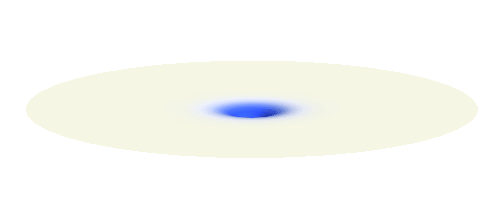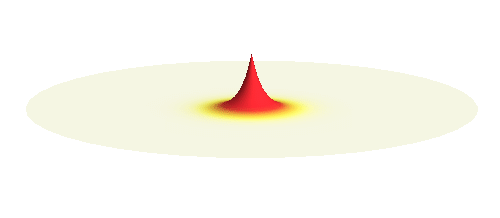
What if we ask an electron about its position? Such measurement also changes the electron wave. In this case it instantaneously collapses into a wave that has a well-defined position: A very thin peak localized around some point in space. The probability that the wave collapses into a specific point in space is proportional to the square value of the wave at that point. A quick look at the animations above reveals that the 1s electron is most likely to be found in the center because the wave has its maximum there. On the other hand, the 2p_x orbital is never found in the center because the wave has zero amplitude there. It is the spatial extent of the electron wave why it is sometimes claimed that an electron can be “at several places at once”.
And what happens when we ask an electron about its momentum (i.e. mass times velocity)? This time we have to identify states with well defined momentum. They turn out to be the ordinary sine waves with all possible wavelengths and propagating in all possible directions; the first represents the magnitude and the second the direction of the momentum. There is a mathematical theorem (the Fourier decomposition) according to which any wave can be expressed as certain combination of suitably chosen sine functions. The details are technical but we it is feasible to understand it without math. Have again a look at the 1s and 2p_x waves animated above. The peak of the 1s orbital is narrower than the peaks of the 2p_x orbital. This means that the first will be well fitted by sine waves with smaller wavelength than the second. Smaller wavelengths mean larger momentum. (Do you remember that light with short wavelengths is more energetic and carries more momentum? The same statement holds for electron waves.) This means that 1s electrons move on average faster than 2p_x electrons.
We can finally address the almost mystical uncertainty principle. The electron cannot have a well-defined position and momentum at the same time. The first requires the electron wave to be peaked at tiny extent of space, while the second requires it to be an extended sine function. These two conditions are clearly incompatible. This is the essence of the Heisenberg’s uncertainty principle.
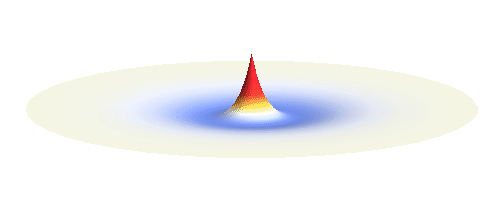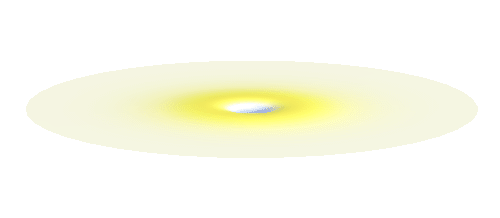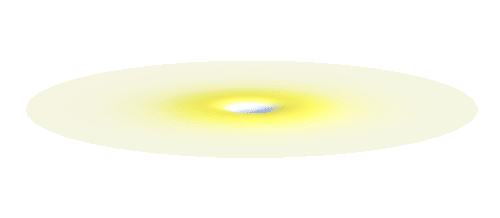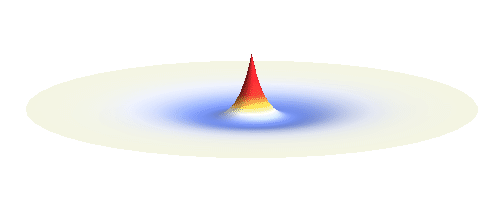
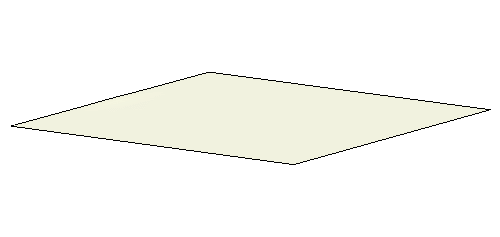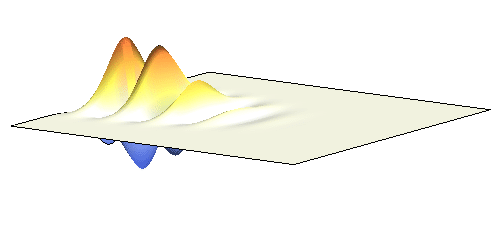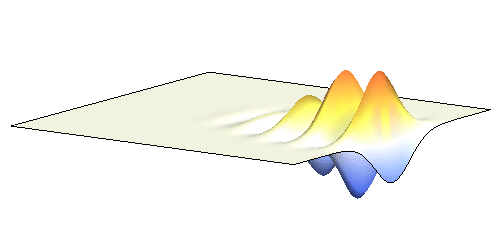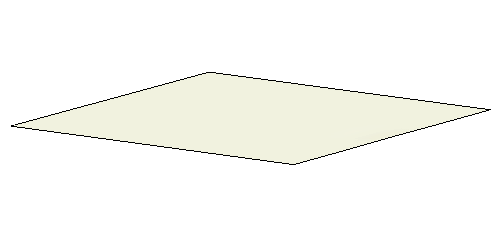
It is possible to make a compromise between position and momentum that is called a wave packet. In that case the electron is not located at one spot but is spread in a wider “packet”. The packet has a sine-function profile but its amplitude decays away from the middle. This is the kind of wave you have to consider in the double-slit experiment. A typical wave packet is shown below.
Now you can visualize a particle in the Wonder World. But can we also visualize several particles in the same way? As mentioned in the previous post, a typical number of particles in a typical object is mind-bogglingly huge, so the question is important. We will see in two weeks that we can do so if we adopt one extra rule. This rule comes in two variants depending on the particle species. We will see that the rule that applies for electrons easily explains why there are so many elements in the periodic table, and also why some materials are insulators while other are metals. The second variant applies e.g. for helium atoms and it explains their superfluid phase characterized by zero viscosity flow. We will get to these phenomena soon!